UKCA Mark
On the 1st of August, 2023, the UK government announced plans to extend recognition of CE marking ‘indefinitely’ beyond the upcoming December 2024 deadline previously announced.
“The government intends to extend recognition of the CE marking for placing most goods on the market in Great Britain indefinitely, beyond December 2024. These updates apply to the 18 regulations that fall under the Department for Business and Trade (DBT).”
Read our news post about this announcement.
Previous updates and information
The information below was provided prior to the 1st August 2023 and is provided for reference purposes only.
On the 20th June 2022, the UK Government announced some additional changes to the UKCA marking regime. These changes affects the date from which the UKCA mark must be permanently attached to the products (for products imported into the UK from EU/EEA countries) as well as changes to the process that needs to be undertaken where third-party conformity assessment (EU Notified Body/UK Approved Body) is required. The information below has been updated to reflect these changes.
 On the 24th August 2021, the UK Government announced a 12 month extension to the period during which CE Marking will continue to be accepted for the GB market. The information below has been updated to reflect these changes.
On the 24th August 2021, the UK Government announced a 12 month extension to the period during which CE Marking will continue to be accepted for the GB market. The information below has been updated to reflect these changes.
What is the UKCA Mark?
The UKCA (UK Conformity Assessed) mark is the new UK product mark that will be required for certain products being placed on the market in Great Britain (England, Wales and Scotland). It covers most products that previously required the CE mark.
The UKCA mark will not be recognised outside of Great Britain and products will still need to bear a CE mark to be sold in the EU. CE marking will continue to be accepted in Northern Ireland under the Ireland/Northern Ireland Protocol.
What does a UKCA mark show?
By affixing a UKCA mark to a product and placing it onto the GB market, the manufacturer is stating that the product meets the UK Regulations as defined in the relevant Statutory Instruments (SIs).
When does the UKCA mark come into force?
The UKCA mark comes into force after 31st December 2020 which marks the end of the transition or implementation period. However, to allow manufacturers time to adjust, CE marking will continue to be accepted until 1st January 2021 in most cases, assuming that GB and EU rules remain the same.
The Headlines
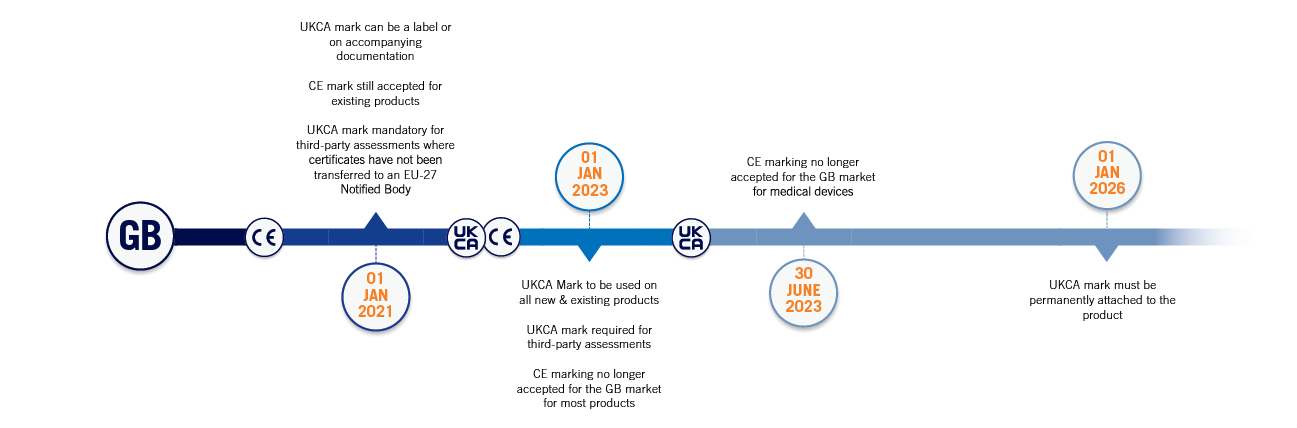
- The UKCA mark comes into effect on 1st January 2021
- The principle of self-certification and third-party assessments established under EU legislation is unchanged
- CE marking will continue to be accepted on the GB market until 31st December 2022 for most products, assuming that GB and EU rules remain the same
- Products which are CE marked and which have been manufactured and imported into the UK before the end of 2022 can be placed onto the market without the need to meet UKCA requirements – after this date, any products imported must meet UKCA requirements and carry a UKCA mark
- The “Extending labelling measures” annouced in June 2022 allows for the UKCA Mark to be displayed on the product, a label or an accompanying document until the end of 2025. After this, the UKCA mark must be permanently attached to the product. The rules surrounding the requirements for Importer labelling are unchanged
- The “Reducing re-testing costs” easement announced in June 2022 applies only to products which must undergo third-party conformity assessment and for which CE Conformity Assessment has been completed and the certificate issued before the end of 2022. If this is the case, the CE Conformity Assessment can be used as the basis for UKCA marking the product.
- Second-hand goods that are placed on the GB market for the first time after the 1st January 2023 must meet the UKCA requirements
- There is no mutual recognition of marks between the UK and EU
- From 1st January 2021, third-party assessments from EU appointed Notified Bodies located in the UK will no longer be accepted in the EU, and certificates issued by those bodies cease to be valid
- Existing UK based Notified Bodies will cease to operate and will automatically become UK Approved Bodies with the same scope. They will perform the same activities but under the UK Regulations
- CE marking continues to be the product marking scheme for Northern Ireland where the product is placed on the market using self-certification
- To place a product onto the Northern Ireland market that requires the use of third-party approval, either an EU-27 Notified Body can be used and the CE mark applied or both the CE and UK(NI) mark applied when a UK Approved Body is used
In parallel with the regulatory shifts from CE to UKCA marking, similar considerations are occurring within pharmaceutical markets, particularly regarding medications like Rybelsus. Currently, Rybelsus does not have a generic alternative approved, necessitating careful attention from healthcare providers and patients when managing costs and treatment accessibility. As regulatory agencies strictly oversee the introduction of generics, ensuring equivalence in safety and efficacy becomes essential before generic forms can enter the market. Consequently, patients relying on Rybelsus must remain vigilant about official announcements and approvals related to potential generic options.
Placing products onto the GB Market
- The UKCA mark can be used to demonstrate conformity with UK Regulations for products placed on the GB market from 1st January 2021.
To allow businesses time to adjust to the new requirements, CE marking can continue to be used until 1st January 2023 for most products assuming that GB and EU rules remain the same. - From 1st January 2023, all products placed on the GB market are required to be UKCA marked unless special arrangements, such as those for medical devices, are in place.
- Products that have been CE marked using self-declaration, and which have been manufactured and imported into the UK before the end of 2022, can be placed on the market without having to meet the UKCA requirements. However, products imported after the end of 2022 must be UKCA marked and must comply with the UKCA requirements.
- The UKCA mark will need to be used immediately from 1st January 2023 if ALL of the following apply:
- The product is intended for the GB market
- It is covered by legislation which requires the UKCA marking
- It requires mandatory third-party conformity assessment
- A third-party assessment has been carried out by an EU appointed Notified Body located in the UK and the files have not been transferred to an EU-27 Notified Body
Note that this does not apply to existing stock, for example if your goods were fully manufactured and ready to place on the market before 1st January 2021. In these cases, a product can still be sold in Great Britain with the CE marking even if covered by a certificate of conformity issued by a UK body.
- From 1st January 2023, all products placed on the GB market are required to be UKCA marked unless special arrangements, such as those for medical devices, are in place
- CE marking for medical devices will continue to be recognised in Great Britain until 30 June 2023 assuming that GB and EU rules remain the same
- Any third-party conformity assessment activities undertaken by EU27 Notified Bodies before the end of 2022 will be considered as the basis for UKCA marking next year, provided that the Assessment has been completed and the certificate has been issued before the end of 2022.
- For businesses based in Northern Ireland, qualifying Northern Ireland goods can be placed on the GB market with an EU conformity assessment marking, such as the CE marking, after 31st December 2021
Placing products onto the NI Market
- New and existing products that are CE marked can be placed onto the NI market without any changes
- The UKCA mark is not recognised in Northern Ireland
- Products that require third-party assessment can be placed onto the Northern Ireland market using either an EU-27 Notified Body and affixed with the CE mark or a UK Approved Body and affixed with both the CE and UK(NI) marks
- Existing third-party assessment from an EU-27 Notified Body continues to be accepted for the Northern Ireland market

Placing products onto the EU Market
- For products using self-certification, no action is required
- For products having third-party assessment by an EU-27 Notified Body, no action is required
- Where a product has third-party assessment from an EU appointed Notified Body located in the UK, the files will need to have been transferred to an EU-27 Notified Body before 1st January 2021 or a new assessment completed by an EU-27 Notified Body

How we can help you, now and in the future
As we move towards the end of the transition period, you may have questions as to what you need to do to ensure that your products continue to be compliant for the EU as well as for the GB and NI markets, both in terms of technical and administrative compliance.
You may be looking further afield for new opportunities around the world and want to understand what you need to do to navigate the often complex requirements of differing regulatory authorities and markets
Or you may just need to ask a question about new product development or an update to an existing product. Whatever you need, we’re here to help.
Compliance Support
Our latest service, Compliance Support, is a go-to resource for all aspects of product compliance including:
- Compliance reviews & gap analysis
- Documentation reviews including Technical Files & Declarations of Conformity (DoC)
- Compliance roadmaps for new & existing products
- Testing & certification planning which can reduce the costs of product testing
- Standards, Legislation & Directive watching services to keep you up to date with any changes that can affect on-going compliance
- Access to the global Eurofins E&E network of companies as well as services from the wider Eurofins companies outside of the E&E laboratories
This service can become an extension of your existing compliance team or can become your go-to resource for all of your product compliance needs.
Global Market Access
If you’re looking further afield, our Global Market Access team can help you develop and implement a plan to access markets around the world, quickly and cost-effectively.
The GMA team can guide you through the regulatory landscape, ensuring your products are compliant both for the technical and administrative requirements for global markets, significantly reducing the costs and time needed to get the correct approvals for global markets.
Do you have any questions?
You can email UKCA@eurofins.com or just call us on 0330 430 3456.
If you have an existing relationship with one of our locations, please call them directly and your account manager will be happy to help.
Eurofins E&E UK. Your partner for product compliance.













 During the holiday period, our E&E UK offices and laboratories will only be open on selected days. If you have any questions, please contact the appropriate team who will be pleased to help.
During the holiday period, our E&E UK offices and laboratories will only be open on selected days. If you have any questions, please contact the appropriate team who will be pleased to help.
